Stem cells – the source of tissue homeostasis
In human tissue, there are various highly differentiated cells specialized to perform distinct functions. For example, there are red blood cells for oxygen delivery, muscle cells for movement, neural cells for signal transduction, etc. These cells are vital for life but they are also prone to various sources of damage since they are all metabolically active. Even worse, they typically cannot renew or replicate themselves.
Fortunately, some cells in the tissue are undifferentiated or only partially differentiated. We call these stem cells. In adult tissue, stem cells exist in a few specific locations in the body (also known as niches). They can further differentiate into different cell types to regenerate the tissue or renew themselves. Notably, the stem cells are maintained in the quiescent stage (i.e., metabolically inactive) most of the time to minimize damage and they are only activated when there is a need to replenish lost cell types in the tissue.

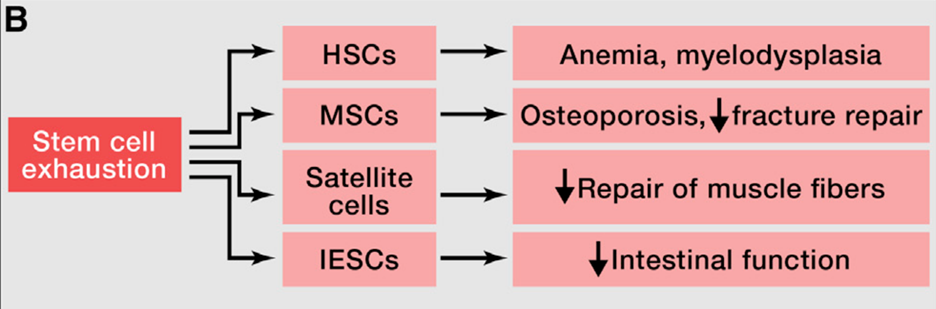
Regenerative potential of stem cells decreases during aging
One of the most pronounced characteristics of aging tissue is decreased regenerative potential. One example would be hematopoiesis (blood cell generation). During aging, the hematopoietic stem cells (HSCs) show a decrease in cell-cycle activity, which means they undergo fewer cell divisions than young HSCs. This results in the decline of hematopoiesis during aging and a reduction in immune cells (also known as immunosenescence). A similar functional decline of stem cells is also found in most tissues, including the brain, bone, and muscle.
Genomic instability accumulated during aging, including damage to DNA and telomere shortening, is one of the leading causes of stem cell decline. Besides that, there are many other sources of damage that can cause a deficient proliferation of stem cells. Therefore, it is important for the stem cells to be maintained in the quiescence stage and avoid the excessive proliferation of stem cells. The previous study in the model organism has shown that the excessive proliferation of stem and progenitor cells leads to exhaustion of stem cell niches and premature aging (Rera et al., 2011, read more at https://pubmed.ncbi.nlm.nih.gov/22055505/).
NAD+ level is crucial for stem cell function
Stem cells rely predominantly on glycolysis for energy, a process that reduces cellular concentrations of NAD+. Mitochondrial function is linked to stem cell maintenance and activation. Induced by aging, mitochondrial dysfunction can result from depletion of NAD+, whereas NAD+ repletion, with precursors such as nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN), can reverse this process.
NAD+ repletion improves muscle stem cell function in aged mice
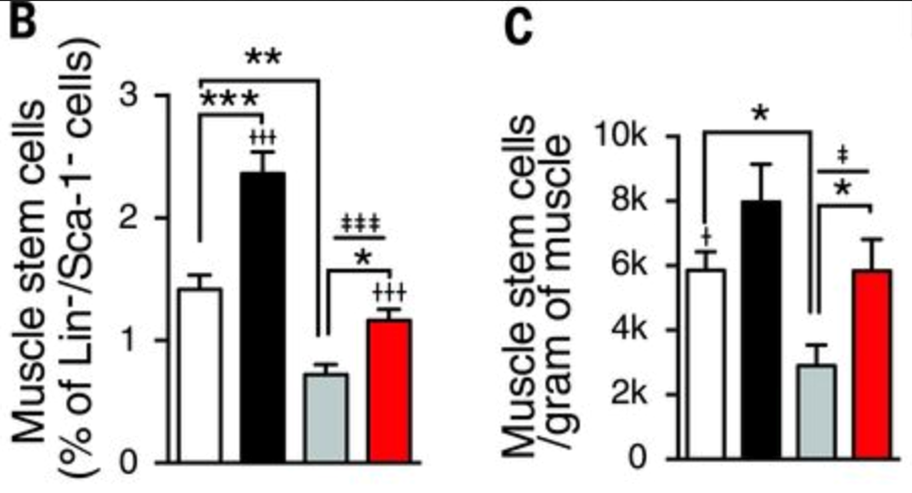
In aged muscle stem cells, the NAD+ level is lower than those in young muscle. Six weeks of NR treatment increased NAD+ concentration in muscle stem cells in both young and old mice. Amounts of NADH were relatively stable. Muscle from aged mice contained fewer muscle stem cells compared with that of young animals. However, NR treatment increased muscle stem cell numbers in young and old mice (Zhang et al., 2016, read more at https://science.sciencemag.org/content/352/6292/1436.full).
NR treatment also enhances muscle function in aged animals through an independent mechanism acting directly on the muscle fibers, as was apparent from improvements in maximal running times and distances, along with limb grip strength in aged mice.
NAD+ repletion increases the regenerative potential of the tissue
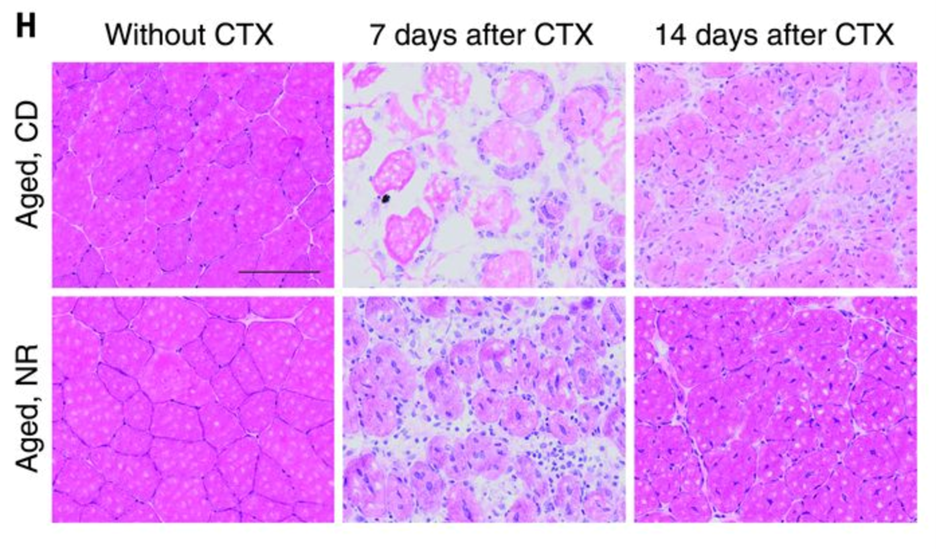
Impairments in muscle regeneration efficiency have been linked to the decline in muscle stem cell function in aged mice. Zhang et al. further showed that the NR treatment accelerated muscle regeneration in aged and young mice cardiotoxin–induced muscle damage. NR-induced improvements in regeneration were paralleled by increases in PAX7-stained muscle stem cells in aged mice. NAD+ repletion also improved the stemness of the aged muscle stem cells. Seven days after CTX-induced damage, aged mice treated with NR exhibited a higher number of muscle stem cells and more recovery from the damage. In addition, according to the study,
“MuSCs were then transplanted from NR-treated or control aged mice into mdx (C57BL/10ScSn-Dmdmdx/J) mice (fig. S2R) (a mouse model of Duchenne muscular dystrophy that gradually loses MuSC function in aging due to the strain of continual muscle regeneration). MuSCs isolated from NR-treated donors more effectively replenished the MuSC compartment and stimulated myogenesis of dystrophin-positive myofibers when transplanted into either young or aged mdx recipients (fig. S2S and Fig. 2L, respectively). Thus, NR treatment can improve both muscle regeneration and MuSC transplantation efficiency.”
NR improves stem cell function in other tissues
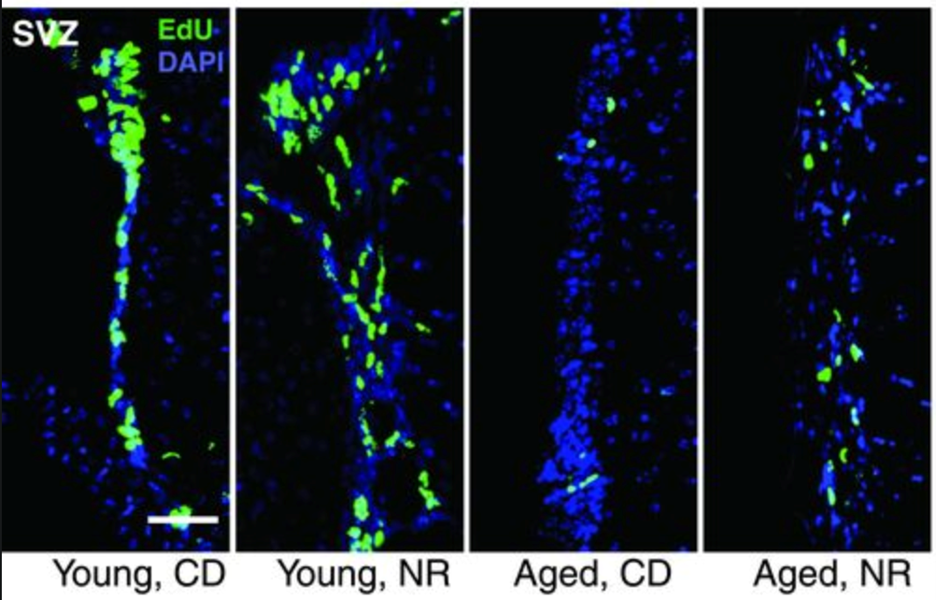
Aging is accompanied by a decline in the number and function of neural stem cells and melanocyte stem cells. NR was also shown to increased proliferation and induced neurogenesis in several brain regions in aged mice. Nicotinamide mononucleotide (NMN), another NAD+ precursor, also has beneficial effects in aged neural stem cells. Similarly, NR rescued the decline of stem cells in hair follicles of aged mice. NR treatment of mice also slightly increased life span.
NMN also promotes neural stem cell function
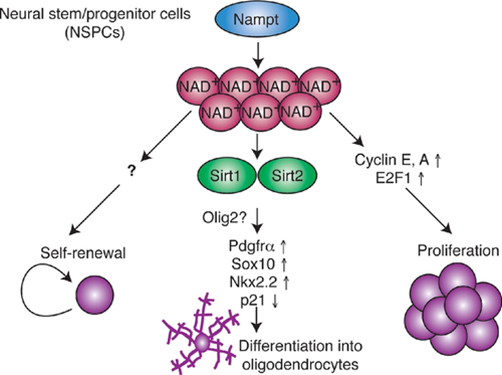
Levels of NAD+ and the NAD+ biosynthetic enzyme Nampt decline in the hippocampus, the brain region that is essential for memory during aging. Nampt ablation in adult neural stem/progenitor cells reduces self-renewal, proliferation, and oligodendrogenesis. Stein and Imai show that age-associated decreases in the stem cell pool are reversed by the administration of nicotinamide mononucleotide (NMN), a key NAD+ intermediate. Nampt-mediated NAD+ bio-synthesis promotes neural stem cell self-renewal, proliferation, and differentiation into oligodendrocytes. While the mechanism by which Nampt promotes neural stem cell self-renewal and proliferation remains unidentified, it involves transcriptional upregulation of cyclins and their up-stream regulator. Nampt-mediated NAD+ biosynthesis likely promotes neural stem cells oligodendrocyte lineage fate decisions by activating Sirt1 and Sirt2, two essential proteins in the Sirtuin family (Stein and Imai, 2014. read more at https://www.embopress.org/doi/full/10.1002/embj.201386917).
Altogether, these studies suggest that the decline of stem cell number and function is a hallmark of aging and is a major cause of decreased tissue homeostasis in the old organism. NAD+ precursors have been shown to be able to recover the stem cell function in old tissue effectively, thus could be used as a potential treatment to reverse age-related stem cell exhaustion. Further studies in primates and clinical trials in humans are needed to elucidate further the power of NAD+ precursors in the rejuvenation of stem cells.
