NAD Precursors Cycle Between Host Tissues and the Gut Microbiome
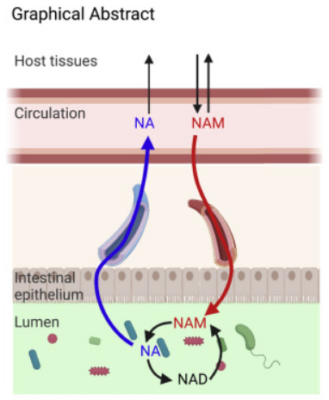
Thus, circulating host NAM enters the gut lumen and is used by the microbiome for both NAD and NA synthesis.
Gut microbiota provide an alternative route from NAM to NAD, bypassing the salvage synthesis pathway, in intestine and liver
Orally delivered NR was found in the GI tract but not in other peripheral tissues, indicating rapid metabolism of NR before it enters the circulation (Figure S4A). Only a small fraction of NAD in the liver and small intestine was M+9, indicating minimal direct assimilation of NR
NR is catabolized to NA by gut microbiota, and the resulting NA supports NAD synthesis in host tissues.
In this study we established that the large intestinal microbiome uses inulin as a dietary source for NAD synthesis.
Thus, circulating host NAM enters the gut lumen and is used by the microbiome for both NAD and NA synthesis.
Gut microbiota provide an alternative route from NAM to NAD, bypassing the salvage synthesis pathway, in intestine and liver
Orally delivered NR was found in the GI tract but not in other peripheral tissues, indicating rapid metabolism of NR before it enters the circulation (Figure S4A). Only a small fraction of NAD in the liver and small intestine was M+9, indicating minimal direct assimilation of NR
An important feature of NAPRT, as compared to NAMPT (NAM-dependent synthesis) is that NAPRT is not subjected to feedback inhibition.
Incubation of the purified enzymes with NAD results in inhibition of NAMPT,65 but not NAPRT,66 and preincubation of cells with NA prevents NAM-dependent NAD synthesis, but not vice versa.
NAD levels can theoretically be increased more by NA than by NAM.
We find that high-dose NR can lead to peak circulating NA concentrations over 50 μM in mice. This concentration is comparable to NA levels in plasma following oral NA intake in humans41,42,43 and thus may be sufficient to transiently activate GPR109A or inhibit DGAT2, mechanisms of action that have not previously been considered relevant for NR. Importantly, if NA-dependent mechanisms play a role in the beneficial effects of NR in mice, it could help explain the lack of translation in some clinical trials—the lower doses used for human studies would be unlikely to support comparable elevation of circulating NA.
Our data, however, conclusively demonstrate that NR contributes to NAD mainly via NA.
Although it was originally suggested to result from deamidation of NAD,47 the discovery that microbes deamidate precursors led to the suggestion that it originates from NA.21 The labeling patterns in our study strongly support the latter hypothesis, as labeling of NAAD matches that of NA and not NAD.
