High-Sensitivity C-Reactive Protein (Hs-CRP) Test
$59.00
Measure Inflammation Levels in your Blood
- Collection Method Finger prick | Fasting NOT required | Physician order Included
- Results in 48 hours after the lab gets your sample
- For Ages 18 and up
- Regulations require we ship to US addresses only | Not available for New York state residents
Why test for hs-CRP from home?
Low-level inflammation can smolder in your body without any symptoms. This can slowly damage blood vessels and other parts of the body over long periods of time.
High-Sensitivity C-Reactive Protein or “hs-CRP” detects low level inflammation that might be missed on a standard CRP test.
This at-home inflammation test will help identify low-level inflammation in your body, which can be a sign of hidden health issues that might otherwise go undetected. Check your hs-CRP levels now to uncover if your immune system is triggered to attack. If your immune system remains activated, then it causes damage throughout the body that can lead to serious health conditions.
Healthy lifestyle changes may help lower your inflammation levels. Regularly monitor your hs-CRP levels as you make changes to ensure you’re on the right track.
Testing Process
- Step 1: Order right here
- Step 2: Test at home & ship it to the lab
- Step 3: Activate your kit and get secure results online
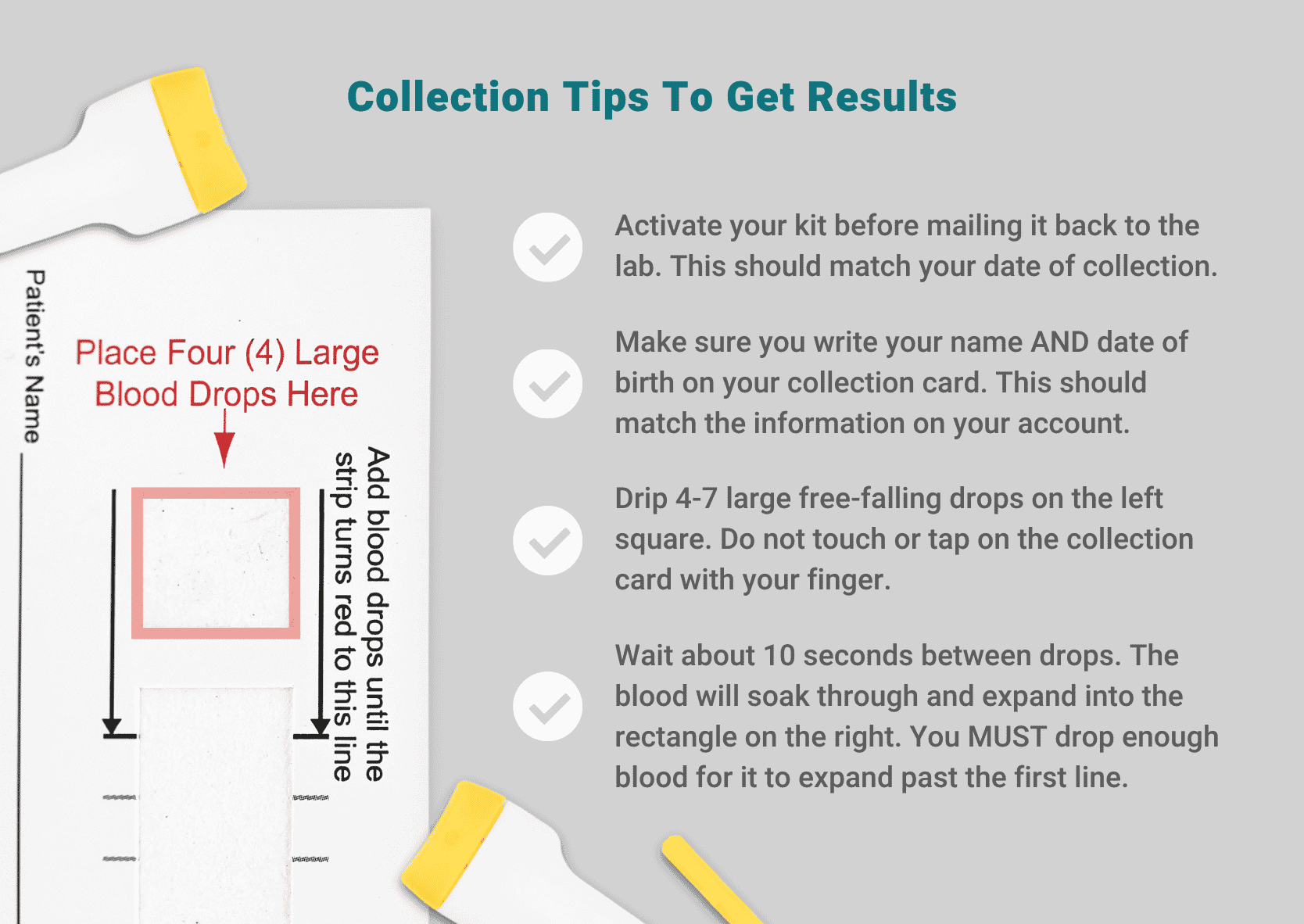
- Activate your kit before mailing it back to the lab. Be sure your account matches the name and date of birth on the collection card. The date you activate your kit should also match your collection date. Only ship your sample back on Monday-Friday before final pickup at your local FedEx drop box.
- It’s essential to get your blood flowing before you collect. Start by running your hands under some warm water. If you’re physically able, perform 5-10 minutes of exercise. Any type of exercise will help get the blood flowing to the end of your finger. Try walking in place, jumping jacks, and/or quick movements of the hand.
- Drip 4-7 large free-falling drops into the collection square on the left. Do not touch or tap on the collection card with your finger. Let blood drip from your finger, about 10 seconds between drops.
- ONLY drop blood on the collection square on the left. The blood will soak through and expand into the rectangle on the right. You MUST drop enough blood for it to expand past the first line.
- Once the blood expands to the first line on the right-hand side of the card, let the sample card dry on a flat surface until completely dry (at least 30 minutes and up to a few hours).
Secure Results
Once your sample is analyzed, we’ll email your results through our secure portal.
Your at-home inflammation test results will be available within 4-7 business days of the lab receiving your completed kit.
This hs-CRP test measures low levels of inflammation, which are considered less than 10 mg/L. If your level is above 10 mg/L, then we recommend consulting a medical professional for further testing.
Get to know your at-home inflammation test
Are you wondering how you’ll use your test results? Not to worry. A detailed Test Guide will be provided with your lab report to help you better understand your hs-CRP test results.
We’ll reveal more about inflammation, what your lab values mean, and action steps for elevated inflammation including a list of anti-inflammatory foods.
All guides are physician-reviewed, and based on peer-reviewed studies in the medical literature including emerging evidence.
Here are the values used by our lab to determine how to categorize your hs-CRP levels:
- Optimal: <1.0 mg/L
- Borderline: 1.0–3.0 mg/L
- Increased Risk: >3.0 mg/L