
There are some very important findings coming out of this research:
- Exogenous NAD+ definitely does cross the blood brain barrier through the Connexin 43 gap junction
- Exogenous NAD+ easily enters the hypothalamus to significantly increase NAD+ levels
- The increased NAD+ in hypothalamus has a strong effect on metabolism, increasing energy expenditure and decreasing hunger
- NMN and NR are not able to cross the blood brain barrier and impact NAD+ levels in the hypothalamus
- More confirmation that extracellular NAD+ acts on a systemic level to increase Sirt1 activity in the hypothalamus which in turn increases metabolism
This study shows that administration of 1 mg/kg of LABELED NAD+ crosses the blood brain barrier to enter the hypothalamus INTACT, increases NAD+ levels, reduces hunger and weight gain, and increases energy expenditure and fat burning in mice.
This study shows that increased NAD+ levels in the blood have a positive effect on metabolic activity in the body.
They also show that NR and NMN can not utilize the cd43 gap to cross the blood brain barrier.
This might explain why NAD+ clinics have found success treating addictions and other brain imbalances, but NR and NMN have not been used in similar fashion.
The positive effect of NAD+ supplementation on metabolism is also unique from that of supplementation with NR and NMN.
Systemic Impact
Elevating NAD+ levels the hypothalamus has great impact throughout the body, as it regulates hunger and energy expenditure.
Even more tantalizing are the possible implications for aging itself. The hypothalamus as master aging clock is a credible theory on aging. Restoring NAD+ levels in the hypothalamus to those of a young animal is very likely to have a positive impact on organs and tissues throughout the body.
NAD+ Decline as we age
Declining NAD+ has been suggested as the central mechanism underlying age- associated weight gain and decline in circadian function [15, 17]. In addition to aging, chronic high-fat, high-sucrose food consumption also reduces hypothalamic SIRT1 protein levels and NAD content in mice, leading to decreased SIRT1 activity in hypothalamic neurons [17].
Decrease NAD+ impacts Sirt1 action
Because of the importance of NAD-dependent SIRT1 activity in normal metabolic function [18], therapeutic strategies activating the cellular NAD-SIRT1 axis have been proposed. Several studies have reported the metabolic benefits of SIRT1-activating compounds including polyphenols such as resveratrol and synthetic small molecule SIRT1 activators [19].
In previous research, NAD+ precursors have been used to increase NAD+
Supplementation with the NAD precursors NMN and NR reportedly exerted beneficial metabolic effects in obese or aged mice by restoring NAD levels in peripheral metabolic organs.
Extracellular NAD can increase internal cellular NAD levels
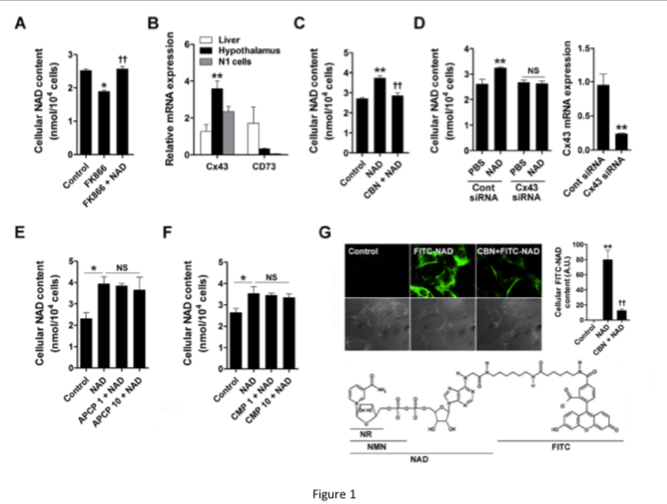
Our study shows that supplementation with NAD effectively increased hypothalamic NAD content in mice.
Decreased hunger
10 week old mice were injected by IV with .14, .7 or 1.4 mg of NAD after fasting overnight.
The .7 dosage was most effective at suppressing food consumption and weight gain when mice were presented unlimited food.
This appetite suppression persisted for 24 hours after NAD+ injection.
In a different experiment, healthy young mice were fasted overnight, then given NAD+ by IP injection of .3, 1, or 3 mg/kg. All 3 groups had significantly decreased food intake over 4 hours. The decreased hunger persisted for 24 hours with the 1 mg/kg dosage.
Increased Energy
Mice injected with .7 ng of NAD+ had increased energy expenditure for 24 hours following injection (Fig. 2D).
In the second experiment, the mice given 1 mg/kg of NAD+ by IP had significantly increased energy expenditure for 24 hours.
Oxygen consumption (VO2) and carbon dioxide production (VCO2) were higher in mice administered with IP NAD than in controls administered IP saline (Fig. 2G).
Switch to fat burning vs carbohydrate
The IP injection of 1 mg/kg resulted in higher RER, which indicates increased preference for burning of fat to supply the body with energy instead of carbohydrate. Overall activity level was not changed. (Fig. 2H and I)
Safe at 100x higher dosage
Two-week IP administration of NAD at a 100 mg/kg did not induce any adverse toxic effects on animal behavior, blood cell count and liver or kidney function.
Effective at much lower dosage than NR and NMN
Notably, the effective dose of IP-administered NAD (1 mg/kg) was approximately 100- fold lower than that of IP NMN [26, 28].
Exogenous NAD+ appears to be more effective at elevating NAD+ in the brain compared to NR and NMN
A recent study reported that orally-administered NMN and NR were mostly degraded to nicotinamide in the liver before entering systemic circulation and a major product of these NAD precursors in circulation was nicotinamide. [39]
Importantly, exogenous NMN or NR did not cross the blood-brain barrier and appeared to increase brain NAD contents by providing nicotinamide to the NAD salvage pathway.
Moreover, the NMN or NR induced increase in tissue NAD levels was lower in the brain than in the liver and kidney.
Advanced age and obesity result in low NAD+ levels in hypothalamus
Hypothalamic NAD contents are significantly reduced in diet-induced obese mice and genetically-obese db/db mice [17].
Brain NAD levels also decreases with age in Wistar rats and humans [38].
Moreover, single central and peripheral administration of NAD suppressed fasting-induced hyperphagia and weight gain without adverse effects, indicating a therapeutic benefit of NAD supplementation in animals and humans with dysregulated energy balance and/or NAD deficit in the central nervous system.
Therefore, further studies are needed to test the therapeutic effects of chronic NAD supplementation on obesity and aging-related weight gain.
exogenous NAD can be directly transported to hypothalamic neurons.
NAD+ levels in the blood likely signal metabolic response in the brain
Although our study focused on exogenous NAD, endogenous NAD can be released from cells [40] and is found in the blood and extracellular fluids of humans and mice [41].
Indeed, blood NAD levels are increased after moderate exercise [41] and in patients with progressive multiple sclerosis [43], implying physiological or pathophysiological mechanisms regulating NAD secretion.
Accumulated evidence also suggests that NAD plays an essential role as an extracellular factor [40].
Exogenous NAD affects energy homeostasis through regulation of Sirt1
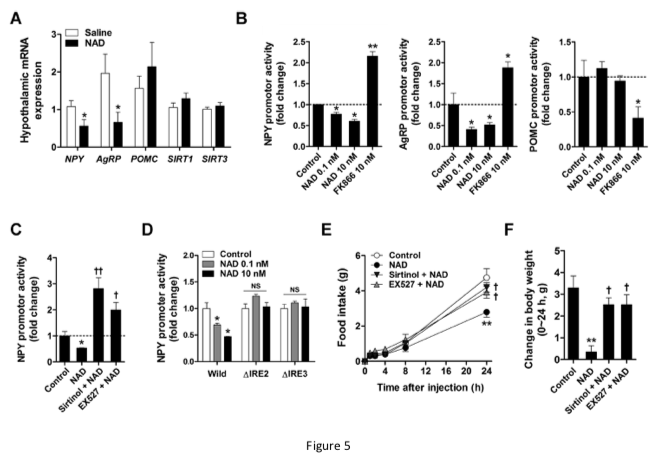
In our work, prior ICV administration of the sirtuin inhibitor sirtinol and SIRT1 inhibitor EX527 significantly inhibited the effects of exogenous NAD on food intake and body weight, suggesting that the actions of NAD were mediated through hypothalamic sirtuins.
Based on our findings and previous findings, we propose a role for Cx43 in hypothalamic NAD transport and the metabolic effect of exogenous NAD (Fig. 6).
In embryonic hypothalamic neuron cells, extracellular NAD was transported into the cells via Cx43 hemichannels, thereby suppressing NPY/AgRP expression through SIRT1/FOXO1.
In mice, exogenous NAD may be transported to the hypothalamus via Cx43 at the blood-brain barrier thereby increasing hypothalamic NAD content and decreasing food intake and weight gain.
Conclusion
In conclusion, our results demonstrate that exogenous NAD is effectively imported into the hypothalamus and increases hypothalamic NAD content. Therefore, NAD supplement can constitute a therapeutic method for metabolic disorders characterized by hypothalamic NAD depletion in humans.



RELATED RESEARCH:
Hypothalamus regulates energy expenditure and hunger (Timper, 2017)
Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity
hypothalamus in particular has emerged as an integrating, superordinate master regulator of whole-body energy homeostasis
In summary, the hypothalamus plays a key role in the regulation of appetite and food intake both in humans and rodents
hypothalamic inflammation impairs the effects of insulin and leptin, contributing not only to hyperphagia and obesity development but also to the associated dysregulation of glucose homeostasis
Brain regulation of appetite and satiety
Energy homeostasis is controlled mainly by neuronal circuits in the hypothalamus and brainstem,
Brain Regulation of Energy Metabolism (Roh, 2016)
The hypothalamus is the region of the brain that controls food intake and body weight
Leptin and insulin signal the status of body energy stores to the hypothalamus.
Hypothalamic regulation of energy homeostasis (Sainsbury, 2002)
these peripheral hormones in ̄uence their e?ects on energy homeostasis either by activating or inhibiting the activity of the orexigenic or anorexic peptides within the hypothalamus
Hypothalamus as master aging clock
Building the Case that Aging is Controlled from the Brain
Is there an Aging Clock in the Hypothalamus?
Hypothalamic programming of systemic ageing involving IKK-b, NF-kB and GnRH (Zhang, 2014)
