Overweight or obese (body mass index 25-42.5 kg/m2), middle-aged and older men and postmenopausal women, 45 years or older.
Participants were asked to take 2 MIB-626 tablets each containing 500 mg of βNMN for 28 days
The randomized participants were asked to take 2 tablets of the study medication orally twice daily for 28 consecutive days. They were asked not to make changes in their dietary intake or to start a weight-reducing diet and were instructed to maintain their usual physical activity during the course of the trial.
-
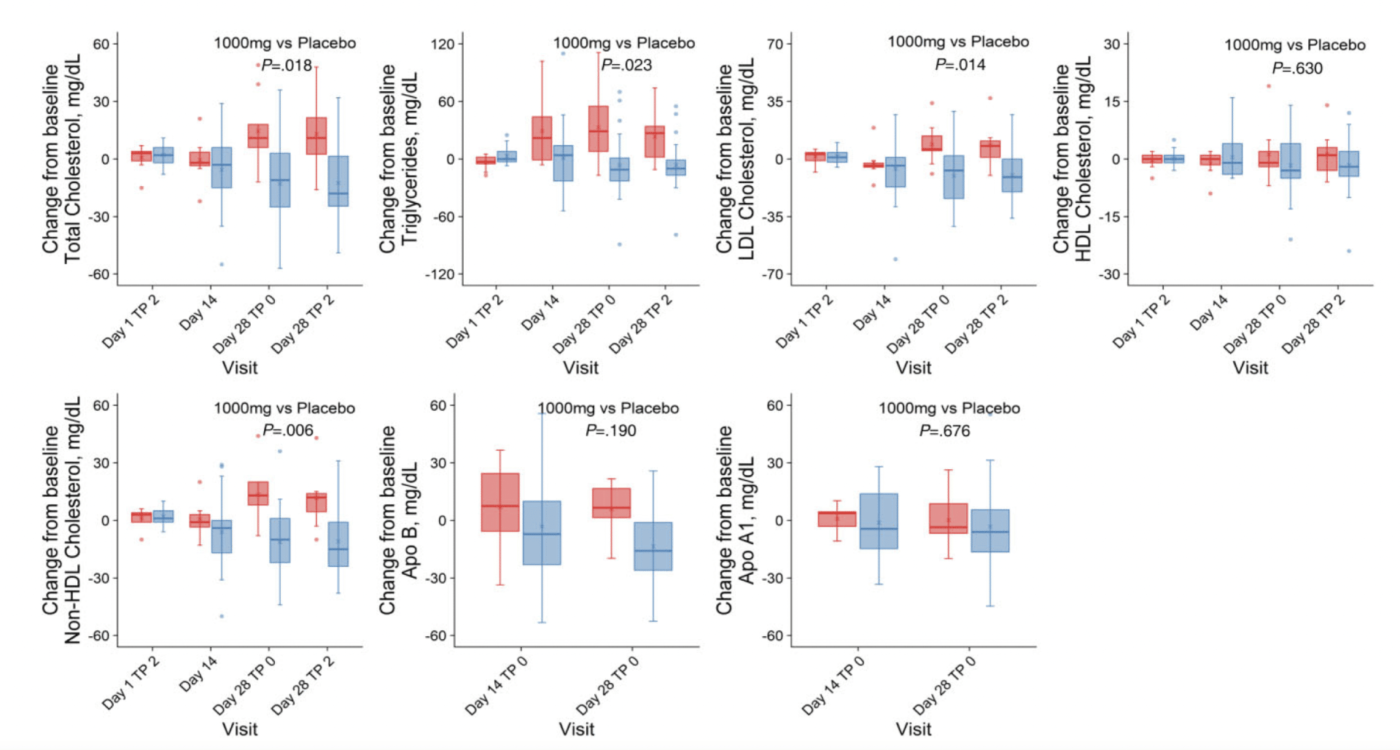
Lower LDL Cholesterol
-
Lower Total Cholesterol
-
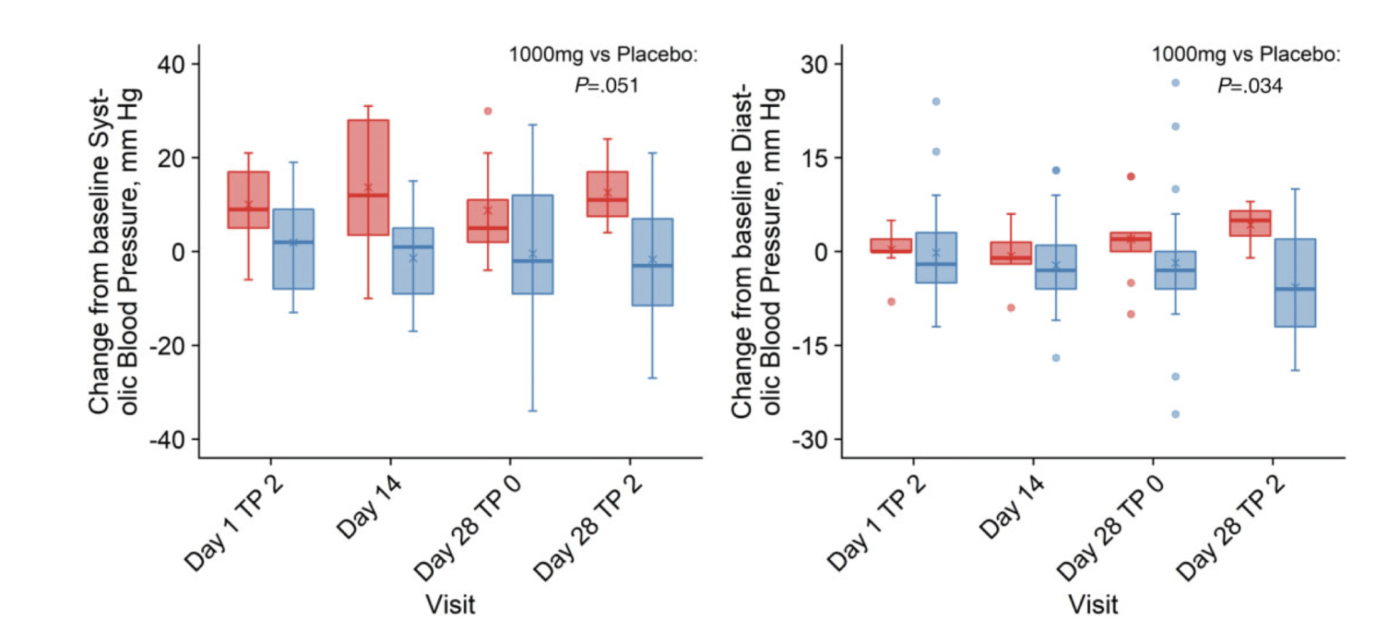
Lower Diastolic Blood Pressure
-
Lower Weight
-
Over 200% increase in NAD+ levels
Some quotes from the study below:
A regimen of 1000 mg of MIB-626 twice daily was safe and efficacious
MIB-626 treatment was associated with a substantial increase in blood NAD level from baseline to days 14 and 28
Circulating NMN levels did not change significantly in either group, except transiently 2 hours after the dose on day 28
Body weight decreased in the participants randomized to the MIB-626 group and increased in those assigned to the placebo group; between-group difference in change in body weight was statistically significant
Serum
A regimen of 1000 mg of MIB-626 twice daily was safe and efficacious
MIB-626 treatment was associated with a substantial increase in blood NAD level from baseline to days 14 and 28
Circulating NMN levels did not change significantly in either group, except transiently 2 hours after the dose on day 28
total cholesterol, low-density lipoprotein (LDL) cholesterol, and non-high–density lipoprotein (HDL) cholesterol levels decreased from baseline in the MIB-626 group
Diastolic blood pressure decreased from baseline in the MIB-626 group
Conclusions: MIB-626 administration in overweight or obese, middle-aged and older adults safely increased circulating NAD levels, and significantly reduced total LDL and non-HDL cholesterol, body weight, and diastolic blood pressure.
